Merck Covid Pill
1 day agoMerck says COVID-19 pill cuts death risk in half will seek emergency authorization. 1 day agoWASHINGTON Drugmaker Merck said Friday that its experimental COVID-19 pill reduced hospitalizations and deaths by half in people recently infected with the coronavirus potentially a leap.
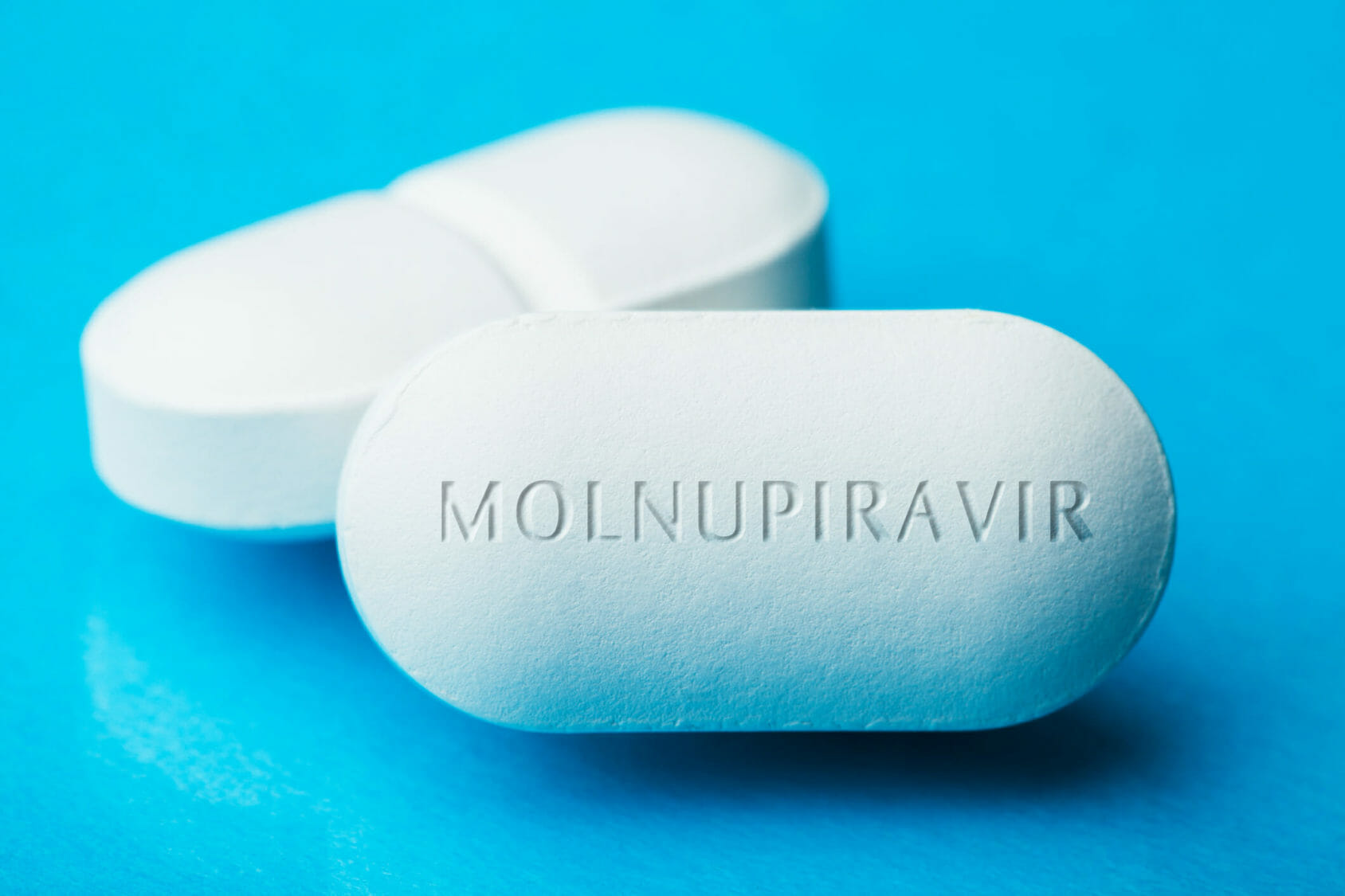
Merck and its partner Ridgeback Biotherapeutics said early results showed patients who received the drug called molnupiravir within five days of COVID-19 symptoms had about half the rate of hospitalization and death as patients who received a dummy pill.

Merck covid pill. 1 day agoMerck and Ridgeback Biotherapeutics said Friday theyve developed a drug that reduces the risk of hospitalization or death by around 50 for patients with mild or moderate cases of Covid. 1 day agoAn investigational antiviral pill reduced the chances that patients newly diagnosed with Covid-19 would be hospitalized by about 50 a finding that could give doctors a. 1 day agoMerck says experimental pill cuts worst effects of COVID-19 Health Canada working to review Mercks experimental COVID-19 pill treatment These 7 symptoms best predict a novel coronavirus.
8 hours agoMerck and Ridgeback Biotherapeutics announced on Friday early result that indicate their experimental oral antiviral drug molnupiravir might halve the risk of death or hospitalization from Covid. 17 hours agoPharmaceutical company Merck Co. 1 day agoMerck on Friday announced that its new pill to treat Covid-19 reduced the risk of hospitalization and death by about 50 percent.
1 day agoMerck will seek US. 1 2021 that its experimental COVID-19 pill reduced hospitalizations and deaths by half in people recently infected with the coronavirus and. 1 day agoMerck is reporting that Molnupiravir a new Covid-19 drug reduces the risk of hospitalization and death.
Approval for pill as soon as possible If approved would be 1st oral antiviral COVID-19 drug Merck shares rally some vaccine makers fall US. Reuters -An experimental antiviral pill developed by Merck Co could halve the chances of dying or being hospitalized for those most at risk of contracting severe COVID-19 according to data that experts hailed as a potential breakthrough in how the virus is treated. Mel EvansAP The pharmaceutical giant Merck on Friday reported good news for people sick.
1 day agoMercks anti-COVID pill cuts hospitalizations 50 in trials. 1 day agoMerck says its new pill reduces deaths by half in new coronavirus patients If cleared Mercks drug would be the first pill shown to treat COVID-19 a potentially major advance in efforts to fight. 1 day agoMerck has said the early results of its trials for its new COVID-19 antiviral pill are significant in that hospitalizations and deaths are halved by the full five-day treatment.
Government to buy 17 mln. Nurse practitioner Wing Wu opens a new packet of vials for blood work at Carbon Health in Oakland Calif. 1 day agoPharmaceutical company Merck Co.
Pharmaceutical company Merck on Friday said it will seek an. The antiviral molnupiravir cut the risk of hospitalization or death in half. Merck plans to seek emergency authorization for the antiviral pills.
1 day agoMerck Co. 1 day agoDrugmaker Merck said Friday that its experimental COVID-19 pill reduced hospitalizations and deaths by half in people recently infected with the coronavirus and that it would soon ask health. 1 day agoThe drug called molnupiravir was developed by Merck and Ridgeback Biotherapeutics and could be the first oral medication specifically approved for the treatment of COVID.
Said Friday that its experimental Covid-19 pill reduced hospitalizations and deaths by half in people recently infected with the coronavirus and that it would soon ask health officials. If it gets authorization molnupiravir which is designed to introduce errors into the genetic code of the virus would be. Said Friday that its experimental COVID-19 pill reduced hospitalizations and deaths by half in people recently infected with the coronavirus.
Merck is conducting a late-stage trial to see if its antiviral pill can prevent COVID-19 infection in addition to the study that showed it can significantly cut hospitalization and death in those. An experimental COVID-19 treatment pill called molnupiravir being developed by Merck Co Inc and Ridgeback Biotherapeutics LP is seen in this undated handout photo released by Merck. 1 day agoMerck Co Incs experimental oral drug for COVID-19 molnupiravir reduced by around 50 percent the chance of hospitalization or death for patients at risk of severe disease according to interim clinical trial results announced on Friday.
1 2021 that its experimental COVID-19 pill reduced hospitalizations and deaths by half in people recently infected with the coronavirus. 9 hours agoResults from a clinical trial show that Mercks antiviral pill for COVID-19 is effective the company said Friday. 1 day agoWashington Merck Co.

Pill For Covid Works Says Merck 2m School Kids Vaping Sexual Abuse At Who Medpage Today
Pfizer Merck Start Testing Covid Prevention Pills In Late Stage Trials Clinical Daily News Mcknight S Long Term Care News

Merck Mrk Molnupiravir Pill Could Change The Fight Against Covid Bloomberg

A Daily Pill To Treat Covid Could Be Just Months Away Scientists Say

U S Pumps 3 2 Billion Into Covid Fighting Antiviral Effort Bloomberg












/do0bihdskp9dy.cloudfront.net/10-01-2021/t_74000fd4b8f54574b62124f728f16b5c_name_file_1280x720_2000_v3_1_.jpg)
Post a Comment for "Merck Covid Pill"